Gastrointestinal Cancer Early Detection
* This page is only updated periodically (last update 1/18/2025), please see our Publications for most recent work.
Our research group is interested in developing techniques and technologies for improved gastrointestinal cancer screening, including esophageal, gastric and colon cancer. For many of these cancers, early detection is critical for improving patient outcomes, but the current standard of care methods are not effective. Typically, methods such as white light endoscopy or narrowband imaging are used for disease screening with these cancers, but these approaches do not provide sufficient contrast to reliably detect the disease in its early stage (Figure 1) [1]. Our research aims to develop novel optical imaging techniques, primarily label-free imaging methods, for improving early detection.
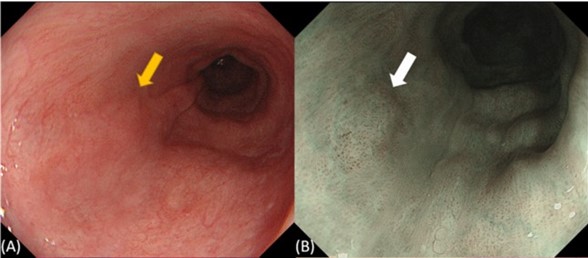
Figure 1. White light endoscopy (WLI) and narrowband imaging (NBI) do not provide sufficient contrast for reliable early detection of gastrointestinal cancers. (A) Endoscopic view by WLI and (B) by NBI of esophageal intraepithelial neoplasia (denoted by arrow). Reproduced from [1].
Recent Work
To identify methods that have high potential for improving disease diagnosis and screening, our work involves conducting tissue testing with ex vivo patient samples to identify optical biomarkers that have high sensitivity and specificity to disease. This includes the development of high order image features such as image texture, and establishing mathematical models for image classification. Towards this end, we have tested a variety imaging methods for different diseases, including, for colon cancer: multiphoton imaging (Figure 2) [2]; for esophageal cancer: polarization imaging, optical coherence tomography, autofluorescence imaging, and hyperspectral imaging [3,4,5]; and for gastric cancer: optical coherence elastography [6,7].
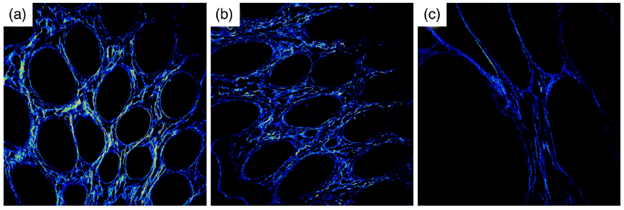
Figure 2: Second harmonic generation images of normal (a), tumor-adjacent (b), and tumor (c) mucosal structures in a single patient. Reproduced from [2].
To move this work toward clinical translation, we also focus on developing miniature imaging instruments for endoscopic implementation of label-free imaging methods. Our work has primarily focused on developing polarization-sensitive endoscopes (Figure 3) [8] as well as multispectral fluorescence endoscopes [9]. In addition to full system development, we are interested in engineering component-level innovations. Collaborative work with Dr. Sarah Bohndiek at the University of Cambridge has focused on developing multispectral filter arrays for compact snapshot spectral imaging [10], and with Drs. Toulouse and Herkommer at the University of Stuttgart has focused on actuation methods in miniature endomicroscopy [11].
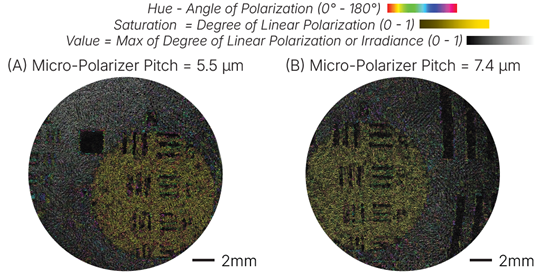
Figure 3: False color polarization Images of MTF imaging target taken with a fiber bundle-base polarization sensitive endoscope using (A) a micro-grid polarizer pitch of 5.5 micons, and (B) a micro-polarizer pitch of 7.4 microns. Reproduced from [8].
Ongoing and Future Work
Gastrointestinal cancers are major public health challenges in urgent need of improved early detection. Our laboratory’s ongoing work continues to optimize the imaging features that we use for disease detection, the mathematical models used to classify our images, and our endoscopic hardware used for in vivo imaging. In the future, we plan to pursue clinical testing of our devices to determine if this technology can improve the standard of care, and how it can best be integrated into the clinical workflow.
References
- Shimoda Y, Shimizu Y, Takahashi H, et al. Optical biopsy for esophageal squamous cell neoplasia by using endocytoscopy. BMC Gastroenterol. (2022)
- Montague J, Young L, Shir H, Sawyer TW, Nfonsam V, Routh J, and Barton JK. Feasibility of non-imaging, random-sampling second harmonic generation measurements to distinguish colon cancer. Biophotonics Discovery. 1(3), 035001 (2024).
- Bonaventura J, Lima N, Alameri A, Bomman S, Banerjee B, Gavini H, Sawyer TW. Assessment of polarized light imaging and optical coherence tomography for improved esophageal cancer detection. Proc SPIE PC12845 (2024)
- Lima N, Bonaventura J, Alameri A, Bomman S, Banerjee B, Gavini H, Routh J, and Sawyer TW. Hyperspectral and auto-fluorescence imaging show promise for detection of esophageal cancer. Proc SPIE PC12854 (2024).
- Bonaventura J, Lima N, Routh J, Alameri A, Bomman S, Banerjee B, Gavini H, Sawyer TW. Exploring Multimodal Imaging for Esophageal Cancer Detection. Biophotonics Discovery. (2025). [In Review]
- Lima N, Kim T, Aitken M, Alameri A, Shivanand B, Rice PF, Banerjee B, Barton J, Sawyer TW. Evaluating optical coherence elastography for detection of gastric cancer and high grade dysplasia by measuring mechanical alterations in ex-vivo human gastric specimens. Digestive Disease Week (2024).
- Gonzales, A, Sawyer TW, Spicer G, di Pietro M, Sanduka A, Rice F, Bohndiek SE, and Barton JK. Optical coherence elastography shows promise for assessing alteration of mechanical properties with onset of gastric cancer. [In Preparation].
- Lima N, DeLeon, C, Sawyer TW. Polarimetry Through a Flexible Imaging Fiber Bundle with a Pixelated Polarizer. Biomed. Opt Exp. (2025). [In Review]
- Slomka B, Duan S, Knapp T, Lima N, Sontz R, Merchant JL, and Sawyer TW. Design, fabrication, and preclinical testing of a miniaturized, multispectral, chip-on-tip, imaging probe for intraluminal fluorescence imaging of the gastrointestinal tract. Front. Biophotonics 3 (2023).
- Taylor-Williams M, Tao R, Sawyer TW, Waterhouse D, Yoon J, and Bohndiek SE. Targeted Multispectral Filter Array Design for Endoscopic Cancer Detection in the Gastrointestinal Tract. J. Biomed. Opt. 29(3), 036005 (2024).
- Wende M, Rothermel F, Stilson E, Kubler F, Sawyer TW, Herkommer A, Toulouse A. 3D-printed endo-microscope with a fast magnetic actuator for axial image plane scanning. Opt. Lett. [In Revision].

