Advanced Optical Microscopy and Imaging
* This page is only updated periodically (last update 1/18/2025), please see our Publications for most recent work.
One of our primary research goals is to advance optical microscopy and other imaging technology in the context of biomedical sciences, with an emphasis on label-free imaging techniques. Within this scope, our interests span a large spectrum of hardware, software and experimental aims. Label-free imaging refers to a subset of imaging technologies that utilize naturally occurring sources of contrast, such as intrinsic tissue fluorescence, birefringence, scattering, or absorption (among others) to measure underlying tissue properties and alterations [1]. Our work most commonly is focused on fluorescence-based modalities and polarization. Natural tissue fluorescence, or autofluorescence, is produced by a variety of different biomolecules (Figure 1), many of which are altered with the onset of disease, making autofluorescence imaging a promising technique for minimally invasive assessment of tissue health [2]. These markers can be measured by cycling through specific excitation-emission wavelength combinations to elicit the different fluorescence processes. While our main interest is in label-free methods, we are also interested in improving technology for labeled imaging such as microscopy with standard histological staining, or through transgenic methods of cell labeling.
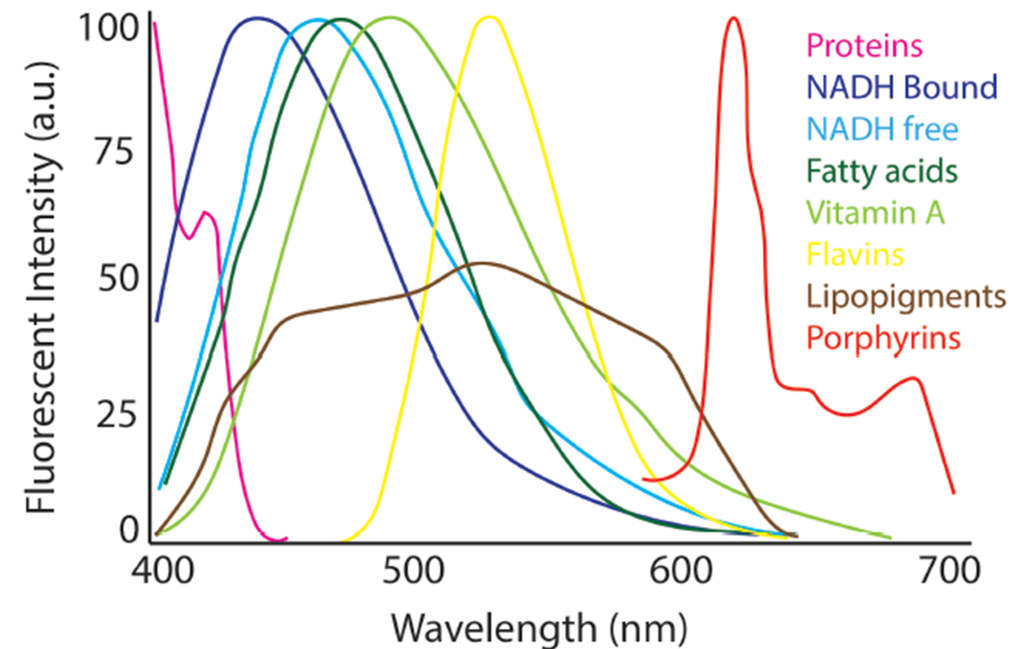
Normalized spectral profiles of endogenous autofluorescent compounds found in tissues for an excitation wavelength of 366 nm. Figure created based on original from [2].
Recent Work
Our recent work includes advancements in image processing for microscopic whole slide imaging platforms, namely tile artifact removal (Figure 2) [3]. In addition, we also focus on image feature extraction for label-free microscopy images with the downstream goal of tissue classification [4,5]. Recently, we have begun to incorporate deep learning into our image analysis pipelines for binary and multi-class classification problems [6].
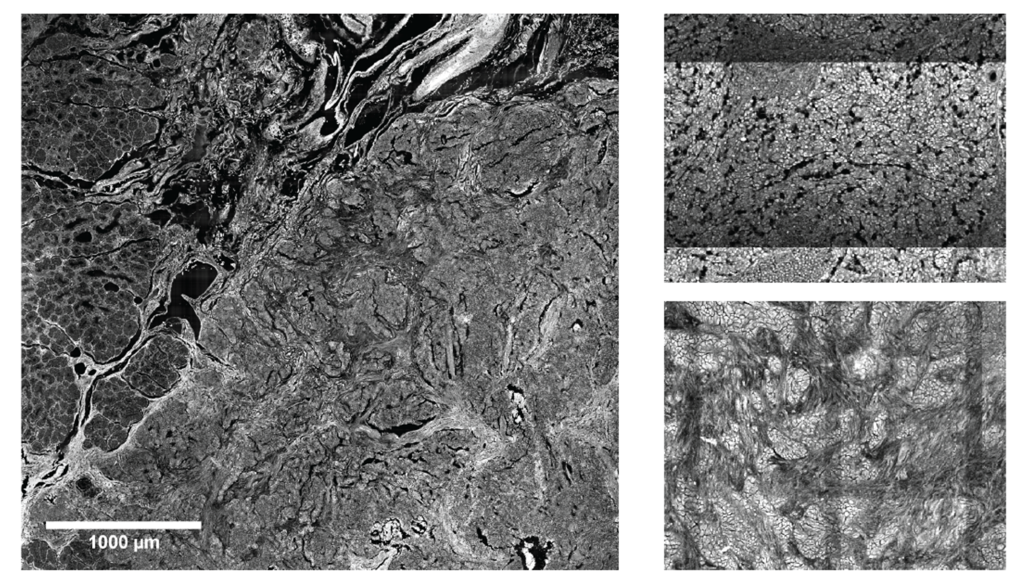
Figure 2. (Left) Whole slide image of duodenal tissue showing the border between normal tissue (left side of image) and tumor tissue on the right hand side. (Right) Examples of different tiling artifact appearances from two separate wavelength channels. All images were collected using two-photon fluorescent microscopy with signal generated from endogenous fluorophores. [3]
More recently, we have begun to investigate the combination of label-free imaging and spatial sequencing platforms such as spatial transcriptomics for the purpose of imaging marker validation, as well as label-free optical phenotyping [7].
Beyond analytics, our work investigates the use of label-free imaging and labeled fluorescence imaging to detect specific tissue processes, for example wound healing using two-photon autofluorescence and second harmonic generation (Figure 3) [8] and organ-level stem cell migration using wide-field fluorescence imaging [9].
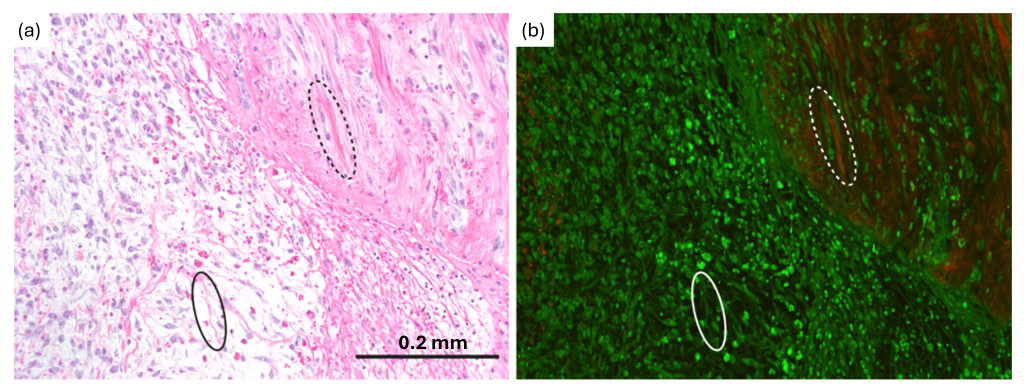
Figure 3. (a) H&E and (b) multiphoton images of a lacerated Achilles tendon showing the wound healing process. Green in multiphoton image is two-photon fluorescence and red is second harmonic generation. Solid oval: fibrin; dotted oval: collagen.
A large research aim in our group investigates the use of polarization imaging for mapping brain microstructure, which is discussed in detail in our dedicated page.
Ongoing and Future Work
Optical microscopy and imaging are used in a wide range of biomedical applications, for example pathology, basic research, and even point-of-care procedures. Much of our ongoing work is focused on identifying label-free biomarkers of clinical or biomedical significance, for example in the context of “label-free staining” or disease diagnostics. In pursuing this goal, we are developing novel image analysis algorithms for feature extraction and classification, both utilizing advancements in deep learning and classical mathematical / statistical modeling. Downstream goals may include the development of novel clinical devices to detect label-free biomarkers.
References
- Shaked NT, Boppart SA, Wang LV, Popp J. Label-free biomedical optical imaging. Nat Photonics. 17(12), 1031-1041 (2023).
- Croce AC and Bottiroli G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. European Journal of Histochemistry 58(4), 320–337 (2014).
- Knapp T, Lima N, Daigle N, Duan S, Merchant JL, and Sawyer TW. Combined flat-field and frequency filter approach to correcting artifacts of multi-channel two-photon microscopy. J. Biomed. Opt. 29(1), 016007 (2024).
- Knapp T, Duan S, Merchant JL, and Sawyer TW. Quantitative Characterization of Duodenal Gastrinoma Autofluorescence using Multi-photon Microscopy. L. Surg. Med. 1-18. (2022).
- Daigle N, Duan S, Merchant JL, and Sawyer TW. Multiphoton microscopy combined with machine learning shows promise for localizing pancreatic neuroendocrine tumors. Proc SPIE PC12846 (2024).
- Guan S, Daigle N, and Sawyer TW. Automated classification of pancreatic neuroendocrine tumors using label-free multiphoton microscopy and deep learning. Proc SPIE 12854 (2024).
- Knapp T, Duan S, Alfonso-Garcia A, Merchant JL, and Sawyer TW. Validation of label-free optical imaging markers of pancreatic cancer using spatial transcriptomics. Proc SPIE 12854 (2024).
- Montague J, Hutchens G, Howard C, Rice P, Besselsen D, Slayton M, Utzinger U, Barton JK, and Sawyer TW. Multiphoton microscopy assessment of healing from tendon laceration and microthermal coagula. L. Surg. Med. (2024).
- Noelle D, Duan S, Song H, Lima N, Sontz R, Merchant JL, and Sawyer TW. Wide field-of-view fluorescence imaging for organ-level lineage tracing of rare intestinal stem cell populations. J. Biomed. Opt. 28(9), 096004 (2023).

