Xiaolin Xu
 My research is about one special protein called rhodopsin, which can be found in our eyes. Actually, rhodopsin serves as an important model of a broad protein family — the G-protein-coupled receptors (GPCR). These receptor proteins deal with interactions of cells with the external environment, as in the case of light, odors and taste, and hormones. Then the activation message is further propagated from GPCRs to activate their cognate G-proteins. The structure of rhodopsin is closely related with its function, which involves light activation. Currently, I am studying the dynamical behavior of one specific high affinity peptide of rhodopsin derived from the C-terminal side of transducin (cognate G-protein of rhodopsin). This research is important in understanding the function of rhodopsin and transducin, which can help us further understand the visual mechanism in our own eyes. The main tool we use is solid-state Deuterium Nuclear Magnetic Resonance (NMR) spectroscopy. From a fundamental level, magnetic resonance methods can tell us about the structure and dynamics of rhodopsin or transducin in a natural membrane environment. My approach will also integrate spectroscopy with diffraction methods and molecular dynamics through investigation of the visual protein rhodopsin. My ultimate aim is to produce a molecular movie of GPCR activation that will be disseminated to the public at large.
My research is about one special protein called rhodopsin, which can be found in our eyes. Actually, rhodopsin serves as an important model of a broad protein family — the G-protein-coupled receptors (GPCR). These receptor proteins deal with interactions of cells with the external environment, as in the case of light, odors and taste, and hormones. Then the activation message is further propagated from GPCRs to activate their cognate G-proteins. The structure of rhodopsin is closely related with its function, which involves light activation. Currently, I am studying the dynamical behavior of one specific high affinity peptide of rhodopsin derived from the C-terminal side of transducin (cognate G-protein of rhodopsin). This research is important in understanding the function of rhodopsin and transducin, which can help us further understand the visual mechanism in our own eyes. The main tool we use is solid-state Deuterium Nuclear Magnetic Resonance (NMR) spectroscopy. From a fundamental level, magnetic resonance methods can tell us about the structure and dynamics of rhodopsin or transducin in a natural membrane environment. My approach will also integrate spectroscopy with diffraction methods and molecular dynamics through investigation of the visual protein rhodopsin. My ultimate aim is to produce a molecular movie of GPCR activation that will be disseminated to the public at large.
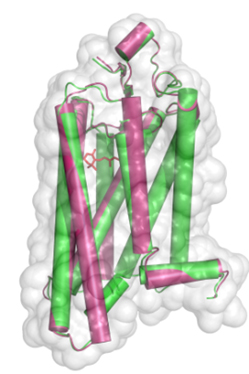
Figure 1: Conformational comparison of active rhodopsin (red) and with ligand-free opsin (green), where alpha-helices are shown as rods within the van der Waals surface of the protein.
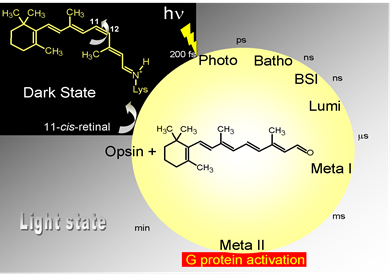
Figure 2: Rhodopsin photoactivation sequence.

