Lauren Slosky
Visualizing Cancer-Induced Bone Pain: A Role for Tumor-Derived Glutamate
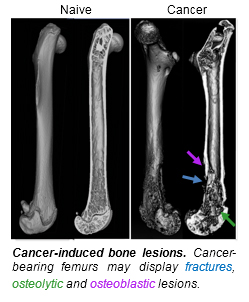
Many common cancers (i.e., breast, prostate, lung) go undetected in their native tissues but cause excruciating pain upon metastasis to bone. Cancer-induced bone pain (CIBP) is a growing health concern, as it is both increasingly common and inadequately managed with current standard-of-care therapeutics. There is a desperate need for novel analgesic agents with efficacy in CIBP as conventional treatments fail with tumor progression. New analgesic targets in CIBP arise as animal models offer insight into this unique and complex pain state. While the etiology of CIBP remains to be fully elucidated, increasing evidence suggests that oxidative stress plays a central role. The pro-nociceptive action of oxidative stress may be attributed, in part, to stress-induced release of the excitatory neurotransmitter glutamate by cancer cells. Glutamate, released from cancer cells, may act on its receptors on primary afferents innervating the bone to activate the fibers and lead to the persistent nociceptive state found in CIBP. Our overarching hypothesis is that tumor-derived glutamate drives CIBP. In the laboratory of Dr. Todd W. Vanderah in the Department of Pharmacology, we are utilizing radiography and confocal microscopy to better understand how glutamate in the bone-tumor microenvironment affects bone integrity and nerve fiber activation. These imaging techniques provide us an unprecedented ability to “see” the disease process and identify mechanisms amenable to therapeutic intervention.