Adam Wallace
 In Andrei Sanov’s physical chemistry group, we study the fundamentals of chemical bonding. Photodetachment of electrons from anions can create highly reactive molecules with two unpaired electrons. The interaction of these two electrons and the atoms around which they are localized is the basis of chemical bonding.
In Andrei Sanov’s physical chemistry group, we study the fundamentals of chemical bonding. Photodetachment of electrons from anions can create highly reactive molecules with two unpaired electrons. The interaction of these two electrons and the atoms around which they are localized is the basis of chemical bonding.
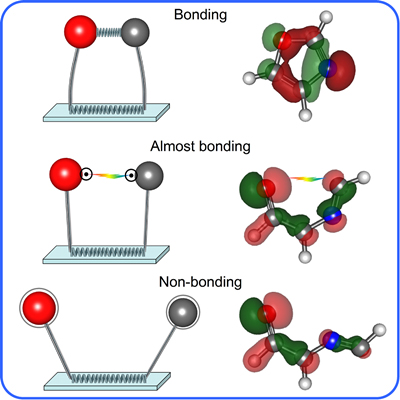
Upon breaking a chemical bond, most molecular fragments quickly separate and no longer interact. However, aromatic molecules have a ring structure, which acts as a tether; this allows us to break a chemical bond and maintain a small distance between the unpaired electrons. By forming molecules in this “almost bonded” state we can probe the energy and structure of extremely short-lived transient species. Earth’s atmosphere is a very energetically-harsh environment in which many molecules have bonds broken and enter short-lived intermediate states en route to their final products. Studying the reactions involving transient species can help us better understand many naturally occurring reactions in the atmosphere as well as the effects of some pollutants.