Research: Lensfree Microscopy & Sensing
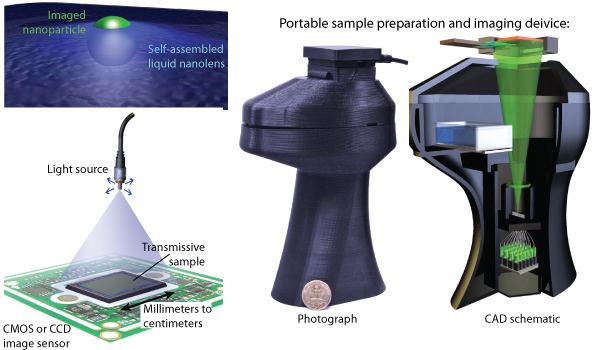
In lensfree microscopy and sensing, a transparent object like a glass slide is placed on top of an image sensor such as that from a cell phone camera or webcam. Diffraction patterns of the object are captured by a computer connected to the image sensor. These patterns are then computationally reconstructed to form an in-focus image of the sample. Unlike conventional imaging, where resolution and field of view are coupled through the numerical aperture of the objective lens, lensfree holographic imaging can provide near diffraction-limited resolution across ultra large fields of view. The lensfree microscopy hardware is also typically much less expensive and bulky than that of lens-based microscopes, making lensfree microscopes suitable for field-portable applications.
Sensing protein biomarkers
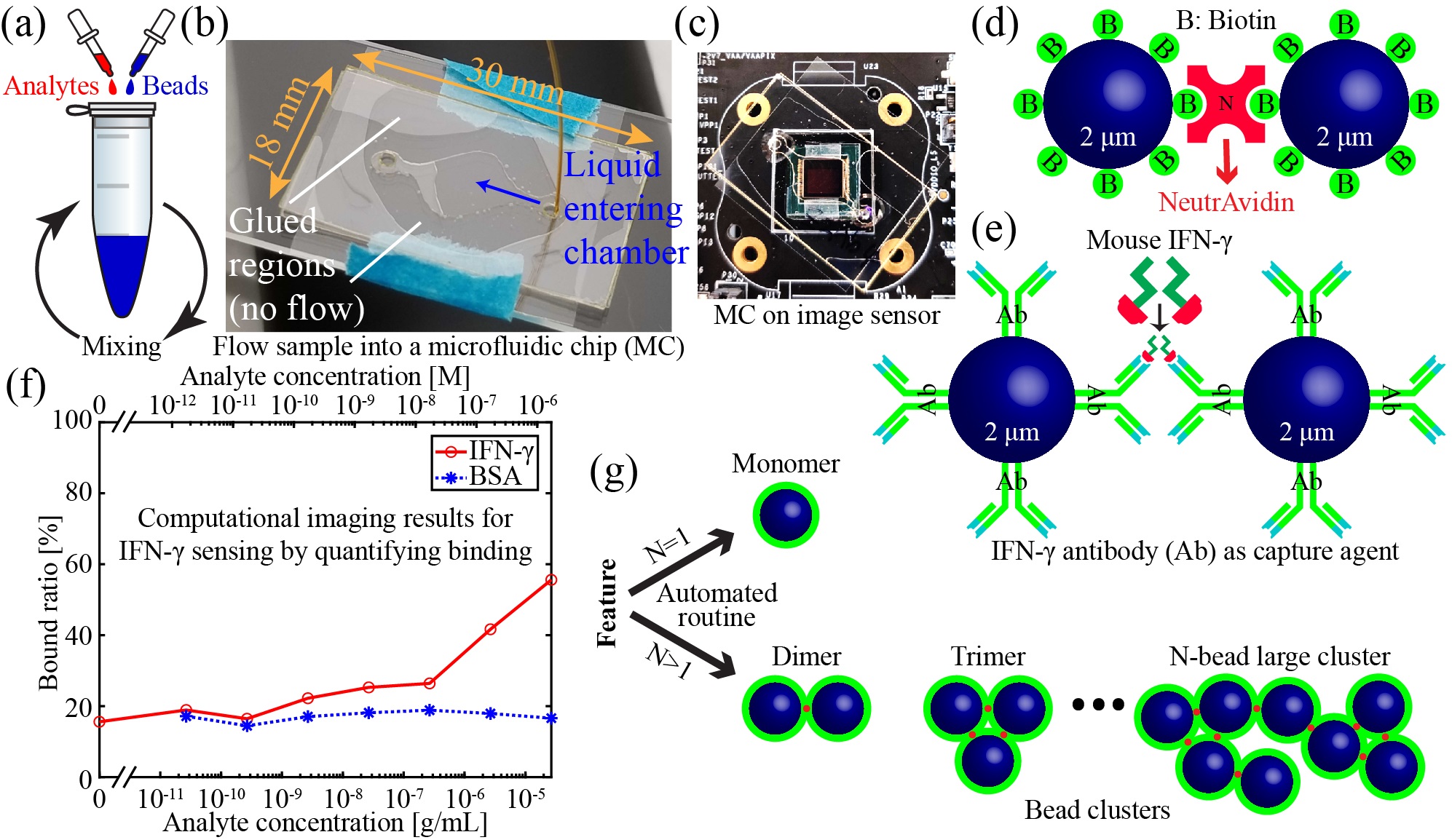
We have combined lensfree holographic microscopy with a bead-based agglutination assay to sense specific proteins in solution in a microfluidic device. Micro-beads are coated with antibodies specific to a target protein. When the protein is introduced to the bead solution, the beads bind to the proteins, causing clusters to form. Automated image processing routines measure the degree of clustering, from which a protein concentration is inferred. The high space-bandwidth product of lensfree microscopy allows >104 beads or bead clusters to be identified and counted in a single image. Such large sample sizes improve the sensitivity and repeatability of the sensor. The low cost and compact size of lensfree holographic microscopes make this approach ideal for point-of-care applications. This approach has similar sensitivity to polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays (ELISAs), but with significantly easier and faster protocols and less expensive hardware requirements. So far, we have used this approach to sense NeutrAvidin, interferon-gamma, and COVID-19.
A few relevant articles include (see publications for all related articles):
- Colin J. Potter, Yanmei Hu, Zhen Xiong, Jun Wang, and Euan McLeod, “Point-of-care SARS-CoV-2 sensing using lens-free imaging and a deep learning-assisted quantitative agglutination assay,” Lab on a Chip, 22, 3744-3754 (2022).
- Zhen Xiong, Colin J. Potter, and Euan McLeod, “High-speed lens-free holographic sensing of protein molecules using quantitative agglutination assays,” ACS Sensors, 6 (3), 1208 (2021).
- Colin J. Potter, Zhen Xiong, and Euan McLeod, “Clinical and Biomedical Applications of Lensless Holographic Microscopy,” Laser & Photonics Reviews, 2400197 (2024).
Pushing the limits of resolution and sensitivity
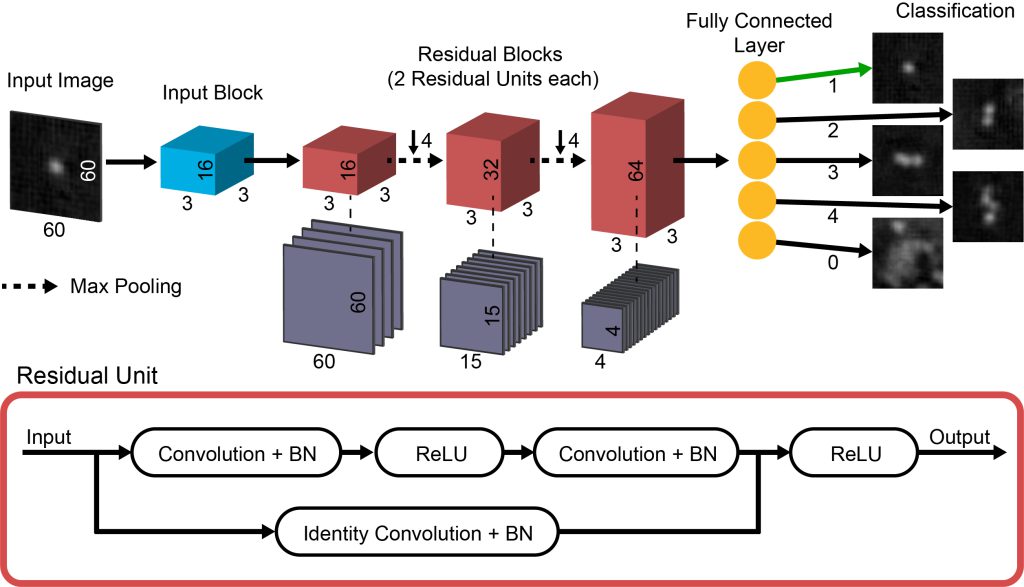
We are combining hardware and computation to improve the ability of lensfree microscopy to image ultra-small objects for nanomateiral characterization, biomedical diagnostics, and environmental monitoring. On the hardware side, we are using time-gated photoluminescence to provide better resolution than conventional filter-based imaging of fluorescent targets. We have also developed a bright and high-speed illumination system for pixel-super-resolved imaging of small particles undergoing Brownian motion in liquids. On the computational side, we are using convolutional neural networks to classify and quantify small targets with high accuracy. Compressed sensing algorithms also provide high-fidelity images of micro-beads. By treating nanoscale objects as a collection of dipole scatterers, the true distribution of materials can be better reconstructed than by simple back-propagation of the electromagnetic field scattered by the materials.
A few relevant articles include (see publications for all related articles):
- Maryam Baker and Euan McLeod, “Lensfree time-gated photoluminescent imaging,” APL Photonics, 8, 061301 (2023).
- Zhen Xiong, Colin J. Potter, and Euan McLeod, “High-speed lens-free holographic sensing of protein molecules using quantitative agglutination assays,” ACS Sensors, 6 (3), 1208 (2021).
- Colin J. Potter, Yanmei Hu, Zhen Xiong, Jun Wang, and Euan McLeod, “Point-of-care SARS-CoV-2 sensing using lens-free imaging and a deep learning-assisted quantitative agglutination assay,” Lab on a Chip, 22, 3744-3754 (2022).
- Colin J. Potter, Shriniketh Sreevatsan, and Euan McLeod, “Deep learning optimization for small object classification in lensfree holographic microscopy,” Optics Express, 32 (20), 35062-35081 (2024).
- Zhen Xiong, Jeffrey E. Melzer, Jacob Garan, and Euan McLeod, “Optimized sensing of sparse and small targets using lens-free holographic microscopy,” Optics Express, 26 (20), 25676-25692 (2018).
- Maryam Baker, Weilin Liu, and Euan McLeod, “Accurate and fast modeling of scattering from random arrays of nanoparticles using the discrete dipole approximation and angular spectrum method,” Optics Express, 29 (14), 22761-22777 (2021).
Self-assembled liquid nanolenses for nanoparticle and virus imaging
Here we combine holographic on-chip imaging with self-assembled liquid nanolenses to image virus-sized particles across ultra-large fields of view of 5 mm x 6 mm and larger. To make this approach sensitive enough to detect individual nanoparticles and viruses, we employ a liquid polymer that self-assembles into nanolenses around the target particles. The main goals of this research are to detect the smallest possible particles across the largest field of view, without the use of labels, and all within a low-cost and field-portable platform. Recently, we have pre-deposited a liquid film on a capture slide before collecting nanoparticles in order to sense ultrafine airborne particulate matter levels in real-time for air pollution monitoring.
A few relevant articles include (see publications for all related articles):
- Maryam Baker, Florian Gollier, Jeffrey E. Melzer, and Euan McLeod, “Lensfree Air-Quality Monitoring of Fine and Ultrafine Particulate Matter Using Vapor-Condensed Nanolenses,” ACS Applied Nano Materials, 6, 13, 11166–11174 (2023).
- Euan McLeod, T. Umut Dincer, Muhammed Veli, Yavuz N. Ertas, Chau Nguyen, Wei Luo, Alon Greenbaum, Alborz Feizi, and Aydogan Ozcan, “High-throughput and label-free single nanoparticle sizing based on time-resolved on-chip microscopy,” ACS Nano, 9 (3), 3265-3273 (2015).
- Euan McLeod, Chau Nguyen, Patrick Huang, Wei Luo, Muhammed Veli, and Aydogan Ozcan, “Tunable vapor-condensed nanolenses,” ACS Nano, 8 (7), 7340-7349 (2014).
- Onur Mudanyali*, Euan McLeod*, Wei Luo, Alon Greenbaum, Ahmet F. Coskun, Yves Hennequin, Cédric P. Allier, and Aydogan Ozcan, “Wide-field optical detection of nano-particles using on-chip microscopy and self-assembled nano-lenses,” Nature Photonics, 7, 247-254 (2013).