Discovering the Fingerprints of Atoms
That’s what teachers call the spectrum of the light atoms emit. Go out after dark and explore the spectra of distant night lights by holding a diffraction grating in front of your eye. You’ll see mostly bands of color, but also some narrow lines. These are direct evidence that atoms have sharp energy levels as described by quantum mechanics.
Unfortunately, what used to be a simple night time adventure just got harder, due to improvements in lamp efficiency. Phosphors have been added to (low pressure Hg) fluorescent lamps so that what you mostly see is bands of red, green, blue, and violet colors.
Even the twisty “green” light bulbs are fluorescent lamps. Low pressure sodium lamps are seldom used for street lighting, leaving the distinctive yellow sodium lamps largely to medical institutions. So today a big challenge is to find old fashioned fluorescent lamps.
If you find an old fluorescent lamp, look carefully at the yellow doublet whose wavelengths are 577 and 579nm. See whether your diffraction grating can resolve the double lines in the yellow (577 & 579nm wavelength).
Modern street lighting is mostly pinkish-yellow high pressure sodium. It’s true that you still see the distinctive black (self absorption) line in the yellow region, but it’s far broader than the low pressure doublet visible in low pressure sodium lamps.
New light emitting diode (LED) flashlights are easy to find, as are red (neon) lasers at checkout scanners in libraries and super markets. Are their line-widths comparable?
New cars are full of LED lamps, some of which blink rapidly. You can see them blinking by moving your eye rapidly. Most car tail lights are filtered incandescent light; others are red LED’s. Can you see the difference?
Check out sunlight and moonlight. (Starlight is too faint without a telescope). What lights can you find around your home? How many different spectra can you find?
Apart from its scientific interest, it’s still thrilling to view from a distance the night lights of a city, or an airport, or a harbor – through a diffraction grating.
References:
- S.F. Jacobs “Night Spectra Quest”, The Physics Teacher, 33, 380, (1995).
- S.F. Jacobs, “Challenges of Everyday Spectra”, J. Chem. Ed, 74, 1070, (1996).
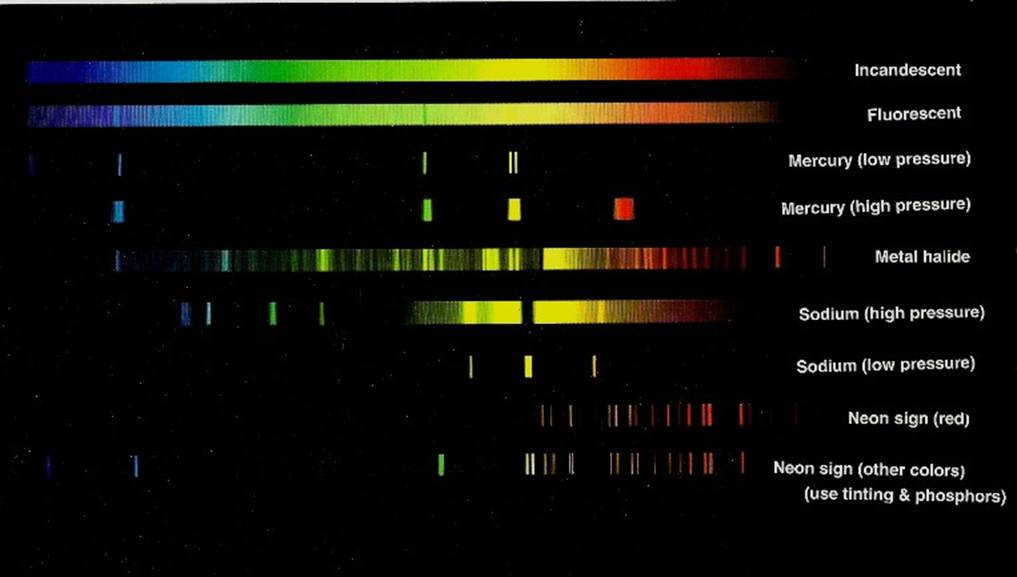
Below is a chart that shows some spectra of night lights.
b) Fluorescent
c) Mercury (low pressure)
d) Mercury (high pressure)
e) Metal halide
f) Sodium (high pressure)
g) Sodium (low pressure)
h) Neon sign (red)
i) Neon sign (other colors, using tinting & phosphors)