Luminescence
Overview: Luminescence occurs when a substance emits light without being heated (eg lightbulbs are heated). Luminescence occurs when a substance absorbs energy from a UV source. Luminescence encompasses both fluorescence and phosphorescence. The difference between the two is the difference in time between energy absorption and emission.
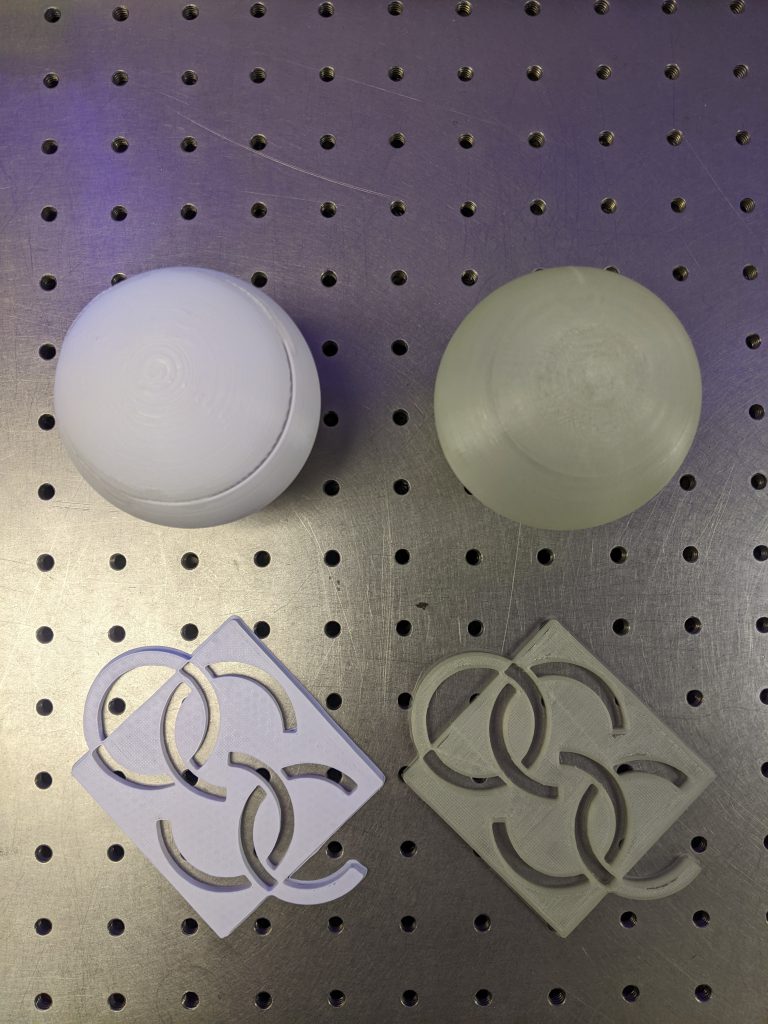
Supplies: Fluorescence items, phosphorescence items, black light
Objectives: Fluorescence and phosphorescence things both glow, but in different ways. How are they different? What are the advantages of each?
Setup:
- Place fluorescence and phosphorescence items on the table
- Plug in and turn on the black light
- Turn off the room lights for better effect
How to run the demo:
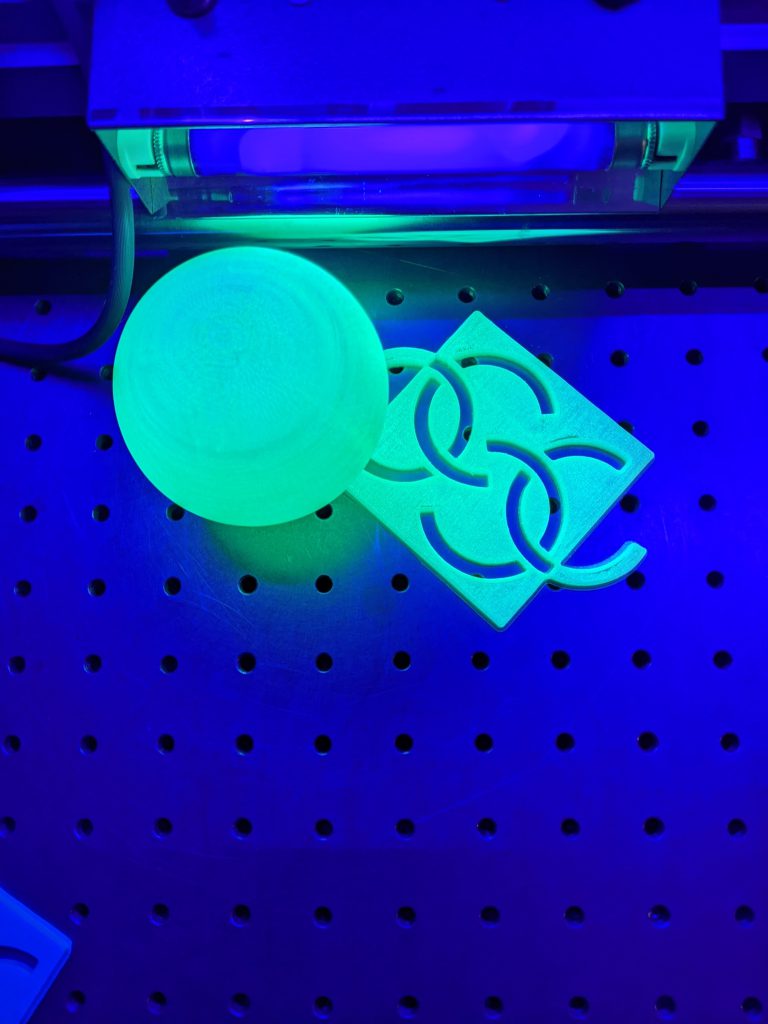
- Expose the different items to the black light
- Show how the fluorescence item stops glowing when the source is removed
- Show how the phosphorescence item still glows when the source is removed





What’s Happening?
Luminescence occurs when an electron in a substance absorbs energy and is excited to a higher energy state. Electrons in this higher state are not stable and will always return to their stable or lower/ground state. In the case of luminescence, the energy being emitted has a longer wavelength and lower energy than the light absorbed. This is why when UV light (that we cannot see) is absorbed, it emits in the visible (a longer wavelength than UV). Fluorescence lasts for short periods of time because the electrons return to their ground state very rapidly after the energy source has been removed. Electrons in phosphorescent material, on the other hand, get trapped in what is called an inner system crossing. The crossed state traps the electrons in an excited state with a much slower release to ground state. This results in the material glowing long after the energy source has been removed.
Try This:
Shine the black light on various items around the room. Do they fluoresce or phosphorece? Try shining the light on white shoes, shoelaces, shirts, sock, etc. Modern detergents contain phosphors to make whites look whiter in sunlight, our largest source of UV light.
Learn more: (external links)

